
Episodes
Thursday May 22, 2025
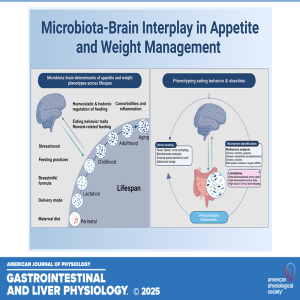
Unraveling Appetite: The Microbiota-Brain Connection Across the Lifespan
Thursday May 22, 2025
Thursday May 22, 2025
Monday Apr 14, 2025
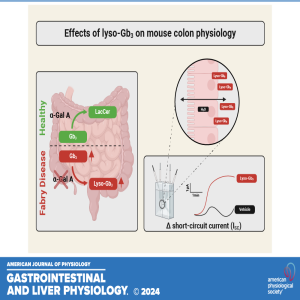
Unraveling Fabry Disease: Lyso-Gb3’s Role in Gut Physiology
Monday Apr 14, 2025
Monday Apr 14, 2025
In this episode, coauthors Cecilia Delprete, Friederike Uhlig, Marco Caprini, and Niall P. Hyland dive into their latest research titled "Characterization of Fabry disease-associated lyso-Gb3 on mouse colonic ion transport and motility."
Fabry disease (FD) is a rare genetic disorder with wide-ranging effects across the body, including the gastrointestinal (GI) system—often one of the first to show symptoms. The team explores how the lipid molecule lyso-Gb3, known to accumulate in FD, affects colonic function using ex vivo techniques like Ussing chambers and organ baths.
The conversation covers key findings: lyso-Gb3 significantly alters ion transport in the colon but doesn't affect motility or nerve responses, suggesting a complex mechanism behind GI symptoms in FD patients. Could this bring us closer to understanding—and eventually treating—the GI discomfort that burdens so many with Fabry disease? Tune in today!
American Journal of Physiology-Gastrointestinal and Liver Physiology 2024 327:6, G810-G817

Thursday Mar 13, 2025
Got Guts: A Chance to Put Liver Biopsy Back to Its Former Glory?
Thursday Mar 13, 2025
Thursday Mar 13, 2025
In this episode, coauthors Camilla Venturin and Luca Fabris delve into their editorial titled "Machine Learning Application to Histology for the Study of Cholangiopathies (BiliQML): A Chance to Put Liver Biopsy Back to Its Former Glory?" The episode explores groundbreaking research by Dominick Hellen and colleagues on the limitations of current histological techniques in studying cholangiocytes and the biliary tree.
Historically, the study of these cells has been constrained by outdated and error-prone methodologies, like two-dimensional cell counting or complex three-dimensional imaging that fails to provide reliable quantification. Enter BiliQML, a novel machine learning model that promises to revolutionize this field. This episode breaks down how BiliQML quantifies biliary forms using anti-Keratin 19 antibody-stained whole slide images, providing a far more accurate and scalable approach.
With an impressive F-score of 0.87, the model's application across a variety of cholangiopathy models, including genetic, surgical, toxicological, and therapeutic, showcases its sensitivity and robustness. The episode reveals how this cutting-edge technology opens new doors for both clinical and basic-science researchers in the field of cholangiopathies. Tune in to discover how machine learning is bringing liver biopsy back to the forefront of research.
American Journal of Physiology-Gastrointestinal and Liver Physiology 2024 327:6, G733-G736
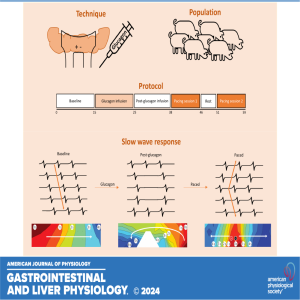
Friday Feb 21, 2025
Friday Feb 21, 2025
In this podcast coauthors Nipuni D. Nagahawatte and Leo K. Cheng discuss their research titled "High-energy pacing inhibits slow-wave dysrhythmias in the small intestine." Glucagon was infused in pigs to induce hyperglycemia and the resulting slow-wave response in the intact jejunum was defined in high resolution for the first time. Subsequently, with pacing, the glucagon-induced dysrhythmias were suppressed and spatially entrained for the first time with a success rate of 85%. The ability to suppress slow-wave dysrhythmias through pacing is promising in treating motility disorders that are associated with intestinal dysrhythmias.
American Journal of Physiology-Gastrointestinal and Liver Physiology 2024 326:6, G676-G686

Thursday Jan 23, 2025
Thursday Jan 23, 2025
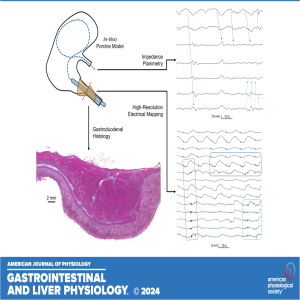
Friday Nov 01, 2024
Friday Nov 01, 2024
Join coauthors Sam Simmonds, Ashton Matthee, and Timothy R. Angeli-Gordon as they delve into their recently published research, "Electromechanical Coupling and Anatomy of the In Vivo Gastroduodenal Junction." In this episode, they explore cutting-edge techniques like electrical mapping, impedance planimetry, and histological analysis to investigate the complex dynamics of the gastroduodenal junction. Discover how contractions in the terminal antrum and pyloric sphincter correlate with gastric slow waves, while the duodenum experiences bursts of spike activity that trigger oscillating contractions. The team discusses their intriguing hypothesis regarding the relative scarcity of myenteric interstitial cells of Cajal in the pylorus, which may hinder coupling between antral and duodenal slow waves.
American Journal of Physiology-Gastrointestinal and Liver Physiology 2024 327:1, G93-G104
Tuesday Oct 15, 2024
Tuesday Oct 15, 2024
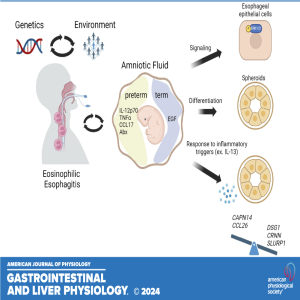
Join coauthors Mark Rochman and Marc E. Rothenberg as they explore their recently published paper, "Amniotic Fluid Modifies Esophageal Epithelium Differentiation and Inflammatory Responses." In this engaging episode, they discuss how the interaction between amniotic fluid and the esophageal epithelium during pregnancy impacts epithelial differentiation and influences the tissue's response to inflammatory stimuli, particularly interleukin 13 (IL-13). Discover the potential implications of these findings, including how they may predispose individuals to inflammatory conditions like eosinophilic esophagitis (EoE) later in life. This episode is a must-listen for anyone interested in developmental biology, immunology, and the long-term effects of prenatal exposures on health.
American Journal of Physiology-Gastrointestinal and Liver Physiology 2024 327:5, G629-G639
Monday Oct 14, 2024
Monday Oct 14, 2024
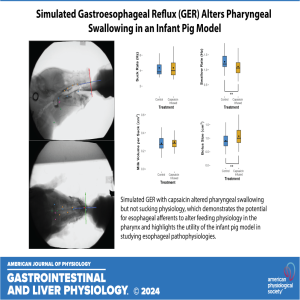
Join coauthor Chloe Edmonds in this insightful episode as she delves into her recently published paper, "The Effects of Simulated Gastroesophageal Reflux on Infant Pig Oropharyngeal Feeding Physiology." Chloe explores how simulating gastroesophageal reflux in an infant pig model reveals significant alterations in pharyngeal swallowing dynamics. Discover how the integration of esophageal afferents impacts motor outputs to the pharynx, leading to decreased swallow frequency and increased bolus sizes. This research not only sheds light on the mechanisms behind esophageal pathologies and oropharyngeal dysphagia but also highlights the potential for developing intervention strategies grounded in neurophysiology. Tune in for a deep dive into the implications of this work for both animal models and clinical practice!
Article Citation:
American Journal of Physiology-Gastrointestinal and Liver Physiology 2024 327:1, G105-G116
Friday Sep 27, 2024
Friday Sep 27, 2024
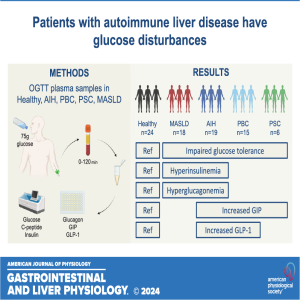
In this episode, coauthors Anne-Sofie H. Jensen and Nicolai J. Wewer Albrechtsen discuss the recently published research titled "Patients with autoimmune liver disease have glucose disturbances that mechanistically differ from steatotic liver disease." The authors reveal that patients with autoimmune liver disease, even in the absence of overt diabetes, experience early glucose disturbances.
Jensen and Albrechtsen highlight key pathophysiological traits specific to these patients, including altered incretin responses. This distinction is crucial because it suggests that the mechanisms underlying glucose metabolism issues in autoimmune liver disease differ significantly from those seen in steatotic liver disease. The authors findings could have important implications for diagnosing and managing glucose dysregulation in patients with autoimmune liver conditions.
Article Citation
American Journal of Physiology-Gastrointestinal and Liver Physiology 2024 326:6, G736-G746
Friday Sep 06, 2024
Friday Sep 06, 2024
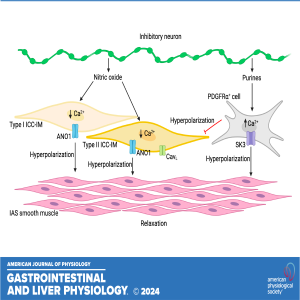
Coauthor Caroline A. Cobine dives into the fascinating world of internal anal sphincter biology in the latest episode of "Gut Guts." The research uncovers direct and indirect neural pathways that modulate intracellular calcium activity in interstitial cells of Cajal, shedding light on their role in smooth muscle cell function.
Article Citation:
American Journal of Physiology-Gastrointestinal and Liver Physiology 2024 327:3, G382-G404
