
Episodes
Friday Jun 21, 2024
Friday Jun 21, 2024
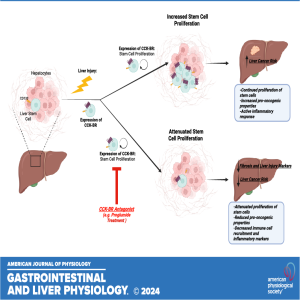
In this episode of "Got Guts" The Micro Version, Jill P. Smith highlights the recently published research titled "Implicating the cholecystokinin B receptor in liver stem cell oncogenesis." This investigation identified a novel pathway involving the activation of hepatic stem cells and liver oncogenesis. Receptor blockade or genetic disruption of the cholecystokinin-B receptor (CCK-BR) signaling pathway decreased the activation and proliferation of hepatic stem cells after liver injury without eliminating the regenerative capacity of healthy hepatocytes.
Article Citation:
American Journal of Physiology-Gastrointestinal and Liver Physiology 2024 326:3, G291-G309
Friday Jun 07, 2024
Friday Jun 07, 2024
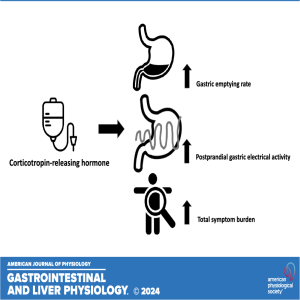
In this podcast, I-Hsuan Huang highlights the recently published paper titled "Effects of corticotropin-releasing hormone on gastric electrical activity and sensorimotor function in healthy volunteers: a double-blinded crossover study." The aim of this research was to investigate the effects of peripheral corticotropin-releasing hormone infusion on meal-related gastrointestinal symptoms, gastric electrical activity, and gastric sensorimotor function in healthy volunteers (HVs).
NEW & NOTEWORTHY In healthy volunteers, peripheral corticotropin-releasing hormone (CRH) infusion accelerates gastric-emptying rate and increases postprandial gastric response, accompanied by a rise in symptoms, but does not alter gastric sensitivity or meal-induced accommodation. These findings underscore a significant link between stress and dyspeptic symptoms, with CRH playing a pivotal role in mediating these effects.
Article Citation:
American Journal of Physiology-Gastrointestinal and Liver Physiology 2024 326:5, G622-G630
Friday May 24, 2024
Friday May 24, 2024
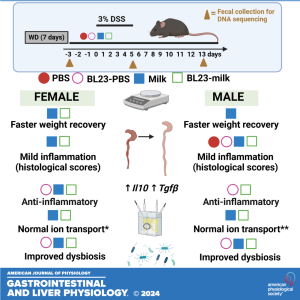
Coauthors Mélanie G. Gareau and Maria L. Marco delve into the findings of their latest research titled "Milk and Lacticaseibacillus paracasei BL23 effects on intestinal responses in a murine model of colitis." This study marked the first exploration into sex-dependent responses to probiotic Lacticaseibacillus paracasei and milk, as well as the potential of the dairy matrix to enhance probiotic protection against colitis. Surprisingly, female mice exhibited greater sensitivity to colonic injury compared to their male counterparts, and neither treatment effectively mitigated inflammation in both sexes. These sex-specific reactions may be attributed to variances in the baseline proportions of Akkermansia in the gut microbiome of female mice, shedding light on the intricate interplay between sex, diet, probiotics, and colitis outcomes.
Article Citation:
American Journal of Physiology-Gastrointestinal and Liver Physiology 2024 326:6, G659-G675
Monday May 13, 2024
Monday May 13, 2024
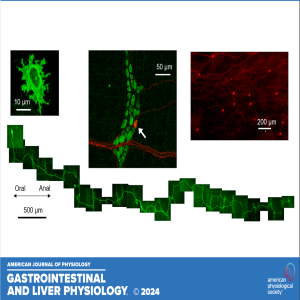
In this episode, Jia Liu and Bin Feng highlight the recently published research titled "Morphological, molecular, and functional characterization of mouse glutamatergic myenteric neurons." In this research, the authors reveal that VGLUT2-positive enteric neurons (EN), although constituting a small fraction of total EN, are homogeneously expressed in the myenteric ganglia, with a slight concentration at the intermediate region between the colon and rectum. Through anatomic, molecular, and functional analyses, we demonstrated that VGLUT2-ENs are activated indirectly by noxious circumferential colorectal stretch via nicotinic cholinergic transmission, suggesting their participation in mechanical visceral nociception.
Article Citation:
American Journal of Physiology-Gastrointestinal and Liver Physiology 2024 326:3, G279-G290
Monday Mar 04, 2024
”Got Guts” The Micro Version: Gastric Neuromuscular Transmission
Monday Mar 04, 2024
Monday Mar 04, 2024
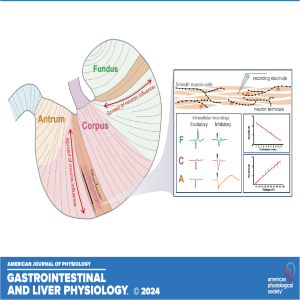
In this episode of the Got Guts podcast series Madeleine R. Di Natale of the University of Melbourne discusses the recently published manuscript titled "Characterization of neuromuscular transmission and projections of muscle motor neurons in the rat stomach." This study provides a detailed mapping of nerve transmission to the circular muscle of the different anatomical regions of rat stomach. It shows that excitatory and inhibitory influences extend around the gastric circumference and that there is a summation of neural influence that allows for finely graded control of muscle tension and length. Nerve-mediated electrical events are qualitatively and quantitatively different between regions, for example, excitatory neurons have direct effects on fundus but not antral muscle.
Article Citation:
American Journal of Physiology-Gastrointestinal and Liver Physiology 2024 326:1, G78-G93
Friday Feb 23, 2024
Friday Feb 23, 2024
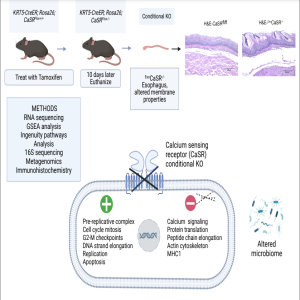
In this episode of the Got Guts Podcast series, Solange M. Abdulnour-Nakhoul of Tulane University, discusses the recently published manuscript titled "Alterations in gene expression and microbiome composition upon calcium sensing receptor deletion in the mouse esophagus." In this research Abdulnour-Nakhoul et al. highlight why calcium sensing receptor plays a significant role in maintaining the barrier function of esophageal epithelium.
Article Citation:
American Journal of Physiology-Gastrointestinal and Liver Physiology 0 0:0

Friday Feb 16, 2024
Friday Feb 16, 2024
Listen as Editor in Chief of AJPGI and Liver Physiology, Mark Frey and the Chair of the Gastrointestinal & Liver Section, Pradeep Dudeja discuss what they are looking forward at the 2024 American Physiology Summit, April 4-7th.
Registration Deadline for American Physiology Summit is March 11, 2024.
Click here for more info on the American Journal of Physiology Gastrointestinal and Liver Physiology
Thursday Feb 08, 2024
”Got Guts” The Micro Version: Pf Inhibits Inflammation-Induced Epithelial Break-Down
Thursday Feb 08, 2024
Thursday Feb 08, 2024
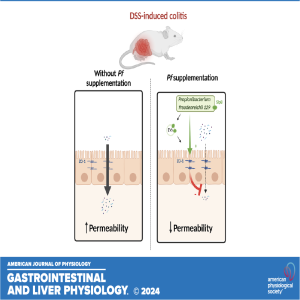
In this episode Marine Mantel of Nantes Université discusses the recently published research in AJP-GI and Liver Physiology titled "Propionibacterium freudenreichii CIRM-BIA 129 mitigates colitis through S layer protein B-dependent epithelial strengthening."
NEW & NOTEWORTHY Propionibacterium freudenreichii reduces dextran sodium sulfate (DSS)-induced intestinal permeability in vivo. P. freudenreichii does not inhibit inflammation but damages linked to inflammation. P. freudenreichii inhibits intestinal epithelial breakdown through S-layer protein B. The protective effects of P. freudenreichii depend on S-layer protein B. Extracellular vesicles from P. freudenreichii CB 129 mimic the protective effect of the probiotic.
Article Citation:
Marine Mantel, Tony Durand, Anne Bessard, Ségolène Pernet, Julie Beaudeau, Juliana Guimaraes-Laguna, Marie-Bernadette Maillard, Eric Guédon, Michel Neunlist, Yves Le Loir, Gwénaël Jan, and Malvyne Rolli-Derkinderen
American Journal of Physiology-Gastrointestinal and Liver Physiology 2024 326:2, G163-G175
Monday Dec 18, 2023
”Got Guts” The Micro Version: IL-4Rα Signaling in Esophageal Smooth Muscle
Monday Dec 18, 2023
Monday Dec 18, 2023
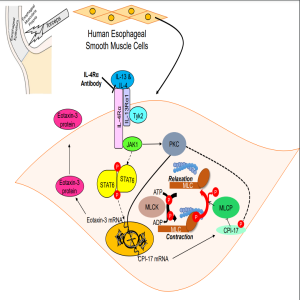
In this episode author, Melissa Nelson of Baylor University Medical Center, discusses the recently published manuscript titled "Th2 Cytokine Signaling through IL-4Rα Increases Eotaxin-3 Secretion and Tension in Human Esophageal Smooth Muscle."
New and Noteworthy:
Nelson et al. have found that Th2 cytokines increase eotaxin-3 secretion and tension in esophageal smooth muscle (ESM) cells via IL-4Rα signaling. Unlike esophageal epithelial cells, ESM cells do not express H+,K+ATPase, and omeprazole does not inhibit their cytokine-stimulated eotaxin-3 secretion or tension. An IL-4Rα blocking antibody reduces both eotaxin-3 secretion and tension induced by Th2 cytokines in ESM cells, suggesting that an agent such as dupilumab might be preferred for EoE patients with esophageal muscle involvement.
Article Citation:
American Journal of Physiology-Gastrointestinal and Liver Physiology 0 0:0
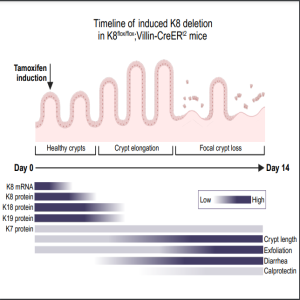
Wednesday Dec 13, 2023
”Got Guts” The Micro Version: Order of Events after Keratin 8 Sequential Downregulation
Wednesday Dec 13, 2023
Wednesday Dec 13, 2023
In this podcast, authors Maria A. Ilomäki and Lauri Polari (both at Åbo Akademi University) discuss their recently published research titled “Defining a timeline of colon pathologies after keratin 8 loss: Rapid crypt elongation and diarrhea are followed by epithelial erosion and cell exfoliation.”
New and Noteworthy:
Understanding the order in which physiological and molecular events occur helps to recognize the onset of diseases and improve their preclinical models. We utilized Cre-Lox-based inducible keratin 8 deletion in mouse intestinal epithelium to characterize the earliest events after keratin 8 loss leading to colitis. These include diarrhea and crypt elongation, followed by erosion and neutrophil activity. The authors results also support noninvasive methodology for monitoring colon diseases in preclinical models.
Article Citation:
American Journal of Physiology-Gastrointestinal and Liver Physiology 0 0:0
